exTra is a Doctoral Network funded by the European Union Horizon Europe Programme. The exTra Consortium is a research network of leading European scientists from academia and industry, experts in clinical transplantation, immunology, pharmaceutical development and medical device manufacture to address key questions on extracorporeal photopheresis (ECP) through a coordinated, interdisciplinary effort. exTra proposes 11 independent doctoral research projects with the ambition of providing its trainees with a comprehensive understanding of basic and translational immunology, especially relating to development and licensing of new immunotherapies. Through its research and training activities, the exTra project will contribute to scientific advancement and innovation in Europe, ultimately leading to societal and economic benefits stemming from clinical innovations in transplant immunotherapy and beyond.
The exTra project is the first consortium dedicated to establishing new indications for ECP in organ transplantation through a deeper understanding of its immunological, pharmacological and pharmaceutical basis. The exTra project brings together leading immunologists, clinicians, pharmacists, bioinformaticians and commercial partners with a common interest in ECP and development of innovative technologies around ECP. Only through the unique and highly-integrated network of expertise assembled by the exTra project will it be possible to achieve the comprehensive biological and pharmacological understanding of ECP necessary to extend its future applications in solid organ transplantation.
Through an
in-depth understanding
of the immunological actions of ECP, exTra aims to:
- Establish a rational basis for extending ECP to novel, personalized indications in solid organ transplantation;
- Define and measure key pharmaceutical qualities (especially potency) of photopheresates;
- Measure pharmacological effects of ECP treatment (eg. changes in myeloid APCs) in transplant recipients;
- Predict short- and long-term clinical effects (eg. reduction of dnDSA levels) in transplant recipients after ECP;
- Improve the patient-tailored delivery of ECP to transplant recipients through optimized treatment regimens.
What is extracorporeal photopheresis?
ECP involves collecting autologous mononuclear cells from blood by apheresis, which are then exposed to 8-methoxypsoralen (8-MOP) and UVA-irradiation to induce apoptotic cell death. The resulting cell suspension, which is known as a photopheresate, is then returned to the patient by intravenous infusion. This procedure not only induces cell death in leukocytes, but also brings blood components into contact with plastic surfaces and anticoagulants, activating cells and modifying plasma proteins. Hence, photopheresates not only contain dead or dying leukocytes, but also a living cell fraction and acellular components.
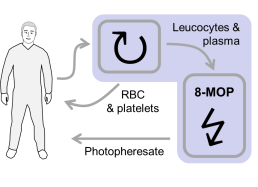
Figure: ECP is a long-established and safe therapeutic procedure. Leukapheresis is performed to collect mononuclear cells (MNC) from peripheral blood of patients. Red blood cells (RBC) and platelets are returned to the patient. Collected MNC are then exposed to 8-methoxypsoralen (8-MOP) and UV-irradiated to induce apoptotic cell death. ECP-treated MNC preparations, which are known as photopheresates, are returned to the patient by intravenous infusion. This procedure not only induces cell death in MNC, but also brings blood components into contact with plastic surfaces and anticoagulants, thereby activating cells and modifying plasma proteins
The immunomodulatory effects of ECP are generally attributed to apoptotic cells or subcellular particles liberated from apoptotic cells. It has been known since the late 1990’s how peripheral tolerance against self-antigens is maintained by a steady-state turn-over of dendritic cells: Non-immunogenic death of parenchymal cells releases self-antigens that are taken up by tissue-resident dendritic cells (DC). These DC then migrate in a semi-matured state to secondary lymphoid organs, where they contribute to self-reactive T cell deletion or anergy, creating an essential niche for Treg survival. ECP harnesses this fundamental mechanism of self-tolerance to promote donor-specific immune regulation in transplant recipients.
Today, ECP is used for special indications in solid organ transplantation, especially retarding bronchiolitis obliterans syndrome (BOS) progression in lung transplant recipients and treating heart transplant rejection. Although these established applications show that ECP is clinically valuable in cardiothoracic transplant recipients, we lack a proper understanding of its immunological actions. Consequently, many clinicians have been reluctant to explore other applications, notably in abdominal organ transplantation. ECP has nevertheless recently resurfaced for various indications, such as minimizing immunosuppression during post-transplant viral infections or malignancy, suppressing DSA levels after kidney transplantation, and avoiding calcineurin inhibitors early after transplantation in patients at high-risk of infection or renal failure.
What diseases are treated with extracorporeal photopheresis?
ECP was originally developed for the management of cutaneous T cell lymphoma (CTCL). Later, it was introduced as an empiric therapy for various autoimmune diseases and inflammatory conditions.
ECP is clearly effective in suppressing T cell-mediated pathologies, mostly notably acute and chronic graft-versus host disease. In experimental models, photopheresates also suppresses contact hypersensitivity, which is a classically T cell-mediated reaction.
On the other hand, ECP doesn’t suppress protective T cell responses; for instance, ECP doesn’t compromise immunity against CMV. Even more surprisingly, ECP actually promotes cytotoxic T cell responses against CTCL and some viral antigens.
ECP reportedly lowers donor-specific antibody levels in heart, lung and kidney transplant recipients. Surprisingly, however, ECP seems not to impair protective antibody responses and it is not an effective treatment for classical antibody-mediated autoimmune dieases, such as myasthenia gravis.
One of the most exciting application of ECP is to treat fibrotic diseases. Recent discoveries suggest that the efficacy of ECP in these conditions may not be entirely due to suppression of T cell responses.
